hERGscreen - Work Packages
WP1 Plant Selection
WP2 Virtual Access
WP3 Extraction, Isolation and Identification
WP4 In vitro hERG assessment
WP5 Pharmacokinetics and pharmacodynamics
WP6 Bioanalytics
WP7 Dissemination
WP8 Management
*********************************************************************************
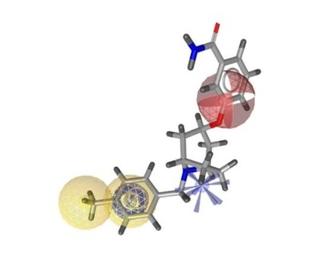
Work Package 1: Plant Selection
Objectives
- Bio-prospection for potentially harmful botanicals from South Africa, South America, and the Mediterranean area
- Selection of potentially harmful botanicals containing relevant amounts of risky constituents
- Supply of selected starting material
Description
The activities in WP1 include a preliminary selection of botanicals with high consumers’ relevance (herbal remedies, foodstuffs, nutritional supplements, spices) with potentially harmful effects in respect to the hERG channel. Emphasis will be put on botanicals available in and originating from the participating countries (South Africa, South America, Mediterranean area), where safety issues are of great importance.
Responsible Partner/s
P9 UFS
*********************************************************************************

Work Package 2: Virtual Access
Objectives
- Rationalized access to potentially harmful botanicals through virtual prediction of putative hERG channel ligands
- Insight into ligand-target interaction
- Virtual ADMET profiling of selected hERG channel blockers
Description
The tasks in WP 2 consist of the application of computational strategies for rationalized, thus time- and cost saving approaches for the prediction of biological and physicochemical properties of constituents of botanicals in order to evaluate their potential cardiotoxic potential and their ADMET properties.
Responsible Partner/s
P1 UNIVIE
*********************************************************************************

Work Package 3: Extraction, Isolation and Identification
Objectives
- Extraction of the selected starting material
- Profiling and micro-fractionation of hERG channel blocking extract
- Isolation and identification of putative hERG-blockers
Description
Based on the rational selection of starting material (WP1, botanicals, preparations, plant species; ~100) the pharmacognostic partners of the consortium will be supplied with the selected materials and will proceed with their proper treatment in order to be extracted. The derived extracts will be screened in vitro on hERG interaction activity revealing potentially harmful botanicals. These will be further scrutinized for the isolation and identification of potential hERG blockers.
Responsible Partner/s
P2 NKUA, P8 UFSC
*********************************************************************************
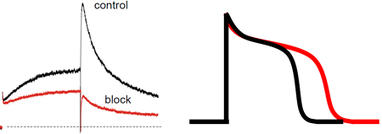
Work Package 4: In vitro hERG assessment
Objectives
- Screening of selected plant extracts for hERG channel inhibition
- Testing of microfractions and establishing activity profiles of separated extracts
- Establishing quantitative data on single compounds
Description
Selected plant extracts will be investigated with respect to hERG channel inhibition on hERG channels heterologoulsy expressed in Xenopus oocytes or mammalian cells. The mechanism of hERG channel block by identified NPs will be studied in more detail.
Responsible Partner/s
P1 UNIVIE, P3 UNIBAS
*********************************************************************************
Work Package 5: Pharmacokinetics and pharmacodynamics (PK/PD)
Objectives
- Rapid high throughput in vivo pharmacokinetic (PK) studies by measuring one PK parameter such as oral area under the curve (AUC) for a series of related compounds to expedite the selection of potential harmful hERG channel blockers
- Assessment of the full plasma-concentration profile in rats after intravenous and oral dosing based on the selection of the previous objective, and evaluation of the relative bioavailability of compounds from corresponding extracts
- In vivo assessment of hERG channel blockage by most potent in vitro hERG-blocking compounds using ECG measurements of the QT-interval in rats
Description
Pharmacokinetic and pharmacodynamics (PK/PD) concepts underlying drug disposition and response provide a quantitative framework with which to identify potential hERG channel blockers. Characterization of a compound’s PK is required to understand how dose level, route of administration, and dosing frequency are related to circulating drug levels and ultimately to concentrations in target tissues. An understanding of PD is required to describe the relationship between drug concentration at the site of action and pharmacologic activity. Thus, during the in vivo hERG screening, PK and PD of potential candidates are characterized in order to define the prospective toxic risks associated with their intake.
Responsible Partner/s
P4 BRFAA, P6 UF, P7 UFRGS
*********************************************************************************
Work Package 6: Bioanalytics
Objectives
- Pharmacokinetic profiling for compounds that are tested in vivo
- Metabolic profiling from urine and plasma samples
- Correlation of these data with biomarkers and PK/PD properties obtained in WP5
Description
Establishment of validated methodologies based on modern analytical approaches for the quantification of bioactive components of botanicals with hERG channel activity. The newly developed methods will be optimized and used for establishing the pharmacokinetic properties of those selected compounds following administration in rodents. In addition, bioanalytical methods (MS and NMR) will be established and applied towards the identification of potential biomarkers associated with the activity of hERG channel inhibitors. Finally, in an effort to further elucidate the mechanism of action of selected hERG channel inhibitors, their metabolism will be investigated based on in vitro incubations with rat and human liver microsomes. The major metabolites derived from those metabolic studies can be further evaluated in terms of hERG channel inhibitory potential.
Responsible Partner/s
P2 NKUA, P4 BRFAA, P6 UF, P7 UFRGS
*********************************************************************************

Work Package 7: Dissemination
Objectives
- Establishment of a scientific network in the field of cardiotoxic botanicals
- Transfer of knowledge between partners
- Promotion of the research activities of the consortium to the scientific community
Description
Transfer of knowledge to all scientists working in the field of NPs and other stakeholders in Europe and America and South-Africa will be achieved through the accomplishment of the hERG screen project by following an active dissemination and exploitation strategy.
Responsible Partner/s
P8 UFSC
*********************************************************************************

Work Package 8: Management
Objectives
- Ensuring the effective coordination and collaboration of partners within the project
- Fulfillment of project activities
- Organization of the annual project meetings
- Preparation and distribution of reports
- Representation of the entire consortium towards the Commission
Description
The particular work package involves all the activities and procedures required for the administration of the project, communication between partners, organization of workshops and meetings.
Responsible Partner/s
P1 UNIVIE
Department of Pharmacognosy
Althanstrasse 14
A-1090 Vienna, Austria
T: +43-1-4277-55255
F: +43-1-4277-855255